Evidence for Maternal Fetal Transmission of Lyme disease
The Lyme disease spirochete, Borrelia burgdorferi, can be transmitted from a pregnant mother, across the placenta, to her baby in the womb. This has been historically reported by the US Centers for Disease Control (CDC), National Institutes of Health (NIH), World Health Organization (WHO) and Canadian Federal Health Authorities.
The US CDC and Health Canada currently acknowledge the risk of maternal-fetal transmission of Lyme disease on their respective websites.
The US CDC and Health Canada currently acknowledge the risk of maternal-fetal transmission of Lyme disease on their respective websites.
Evidence for maternal-fetal transmission of Lyme disease:
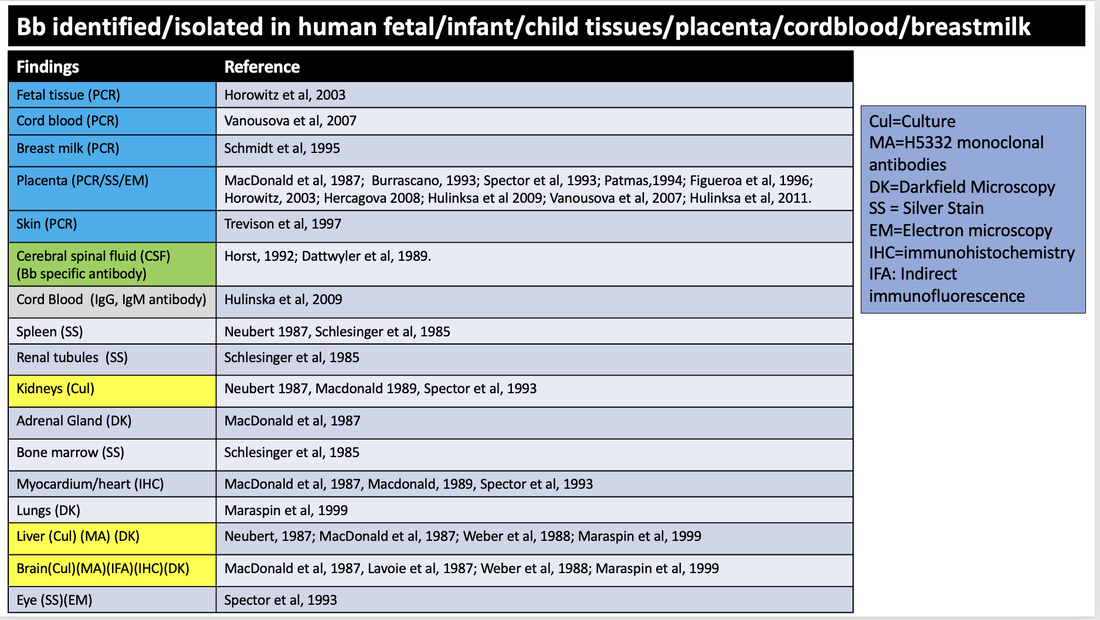
There are 68 cases in which evidence for transmission of B. burgdorferi in pregnancy, from mother to placenta and/or fetus/neonate/child has been described. In two additional cases Bb was identified by PCR in the breastmilk of lactating mothers.
Identification of Bb in fetal, infant, child tissue (22 cases)
1 death of child
8 neonatal deaths
5 cases of stillbirth
8 cases of miscarriage
Live Birth: (18 cases)
15 cases of live-birth, neonatal infection and adverse outcomes
2 cases of live-birth, Bb identified by PCR in cordblood of infants whose mothers were treated
1 case live-birth (twins), one twin had IgG and IgM antibodies (mother was treated)
Placenta: (28 cases)
17 cases Bb identified by PCR in placentas of treated pregnant women
2 cases Bb identified by PCR in placentas of (treatment not stated) pregnancies
4 cases Bb identified in placenta by histology/staining/IFA in untreated pregnancies
4 cases Bb identified in placenta by histology/staining/MaB in treated pregnancies
1 case Bb identifed in placenta by silver stain in pregnancy (maternal treatment not indicated)
Breastmilk:(2 cases)
2 cases whereby Bb has been identified in breastmilk by PCR of women with EM rash
There are 68 cases in which evidence for transmission of B. burgdorferi in pregnancy, from mother to placenta and/or fetus/neonate/child has been described. In two additional cases Bb was identified by PCR in the breastmilk of lactating mothers.
Identification of Bb in fetal, infant, child tissue (22 cases)
1 death of child
8 neonatal deaths
5 cases of stillbirth
8 cases of miscarriage
Live Birth: (18 cases)
15 cases of live-birth, neonatal infection and adverse outcomes
2 cases of live-birth, Bb identified by PCR in cordblood of infants whose mothers were treated
1 case live-birth (twins), one twin had IgG and IgM antibodies (mother was treated)
Placenta: (28 cases)
17 cases Bb identified by PCR in placentas of treated pregnant women
2 cases Bb identified by PCR in placentas of (treatment not stated) pregnancies
4 cases Bb identified in placenta by histology/staining/IFA in untreated pregnancies
4 cases Bb identified in placenta by histology/staining/MaB in treated pregnancies
1 case Bb identifed in placenta by silver stain in pregnancy (maternal treatment not indicated)
Breastmilk:(2 cases)
2 cases whereby Bb has been identified in breastmilk by PCR of women with EM rash
The very first documented case of maternal-fetal transmission of Lyme disease was published in 1985. Authors stated: “The Lyme disease spirochete may also spread transplacentally to organs of the fetus. The mother in this case developed Lyme disease during the first trimester of pregnancy; spirochetes were seen in the spleen, kidney and bone marrow of the infant at term. In addition, the infant had several cardiac abnormalities” "If the infant is ill, the diagnosis of congenital Lyme disease should be considered.’
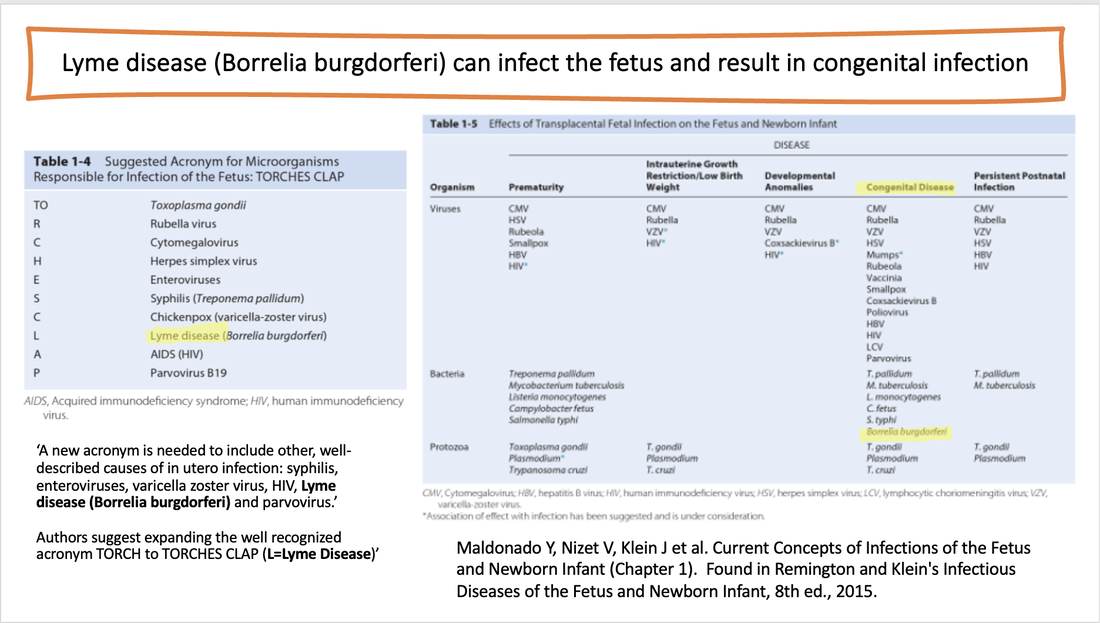
Several groups of experts authored papers reviewing the clinical aspects and spectrum of Lyme disease, also recognizing congenital infection with Lyme disease. One paper stated, “Like syphilis, Lyme disease during pregnancy may be associated with congenital infection of the infant and resulting clinical illness. Several reports describe infants who died shortly after delivery, or were stillborn, to mothers who had Lyme disease during pregnancy. Spirochetes were found in autopsy specimens from some of these babies.”
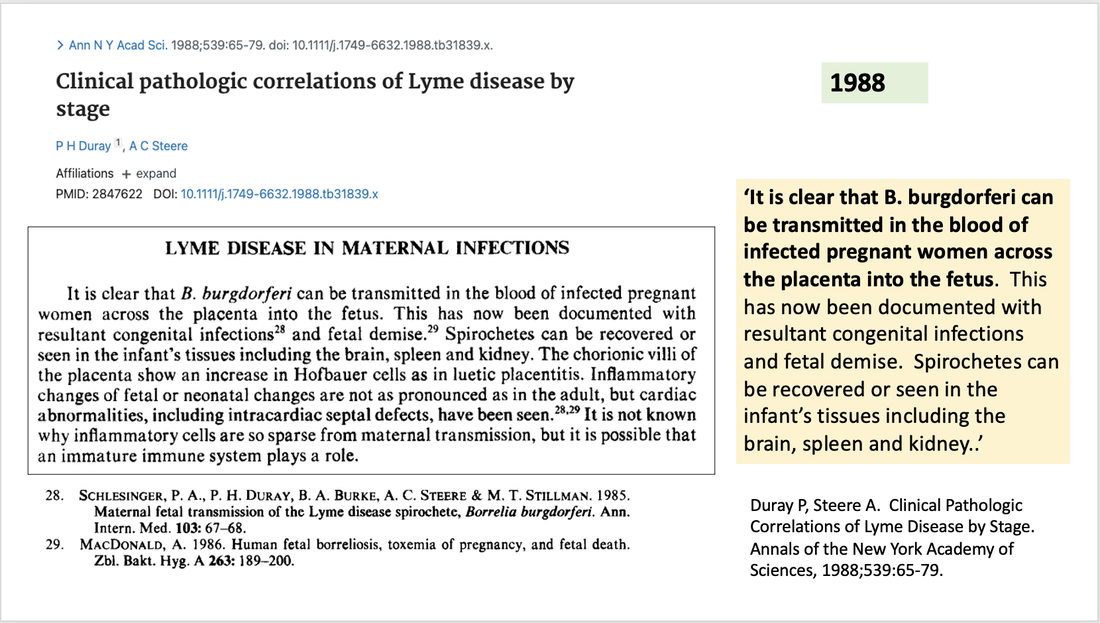
A second review titled Clinical Features of Lyme borreliosis listed congenital disease as part of the main clinical spectrum of Lyme borreliosis, also highlighting similarities to syphilis. A third paper by authors who had also reported on the first documented case of transplacental transmission of Bb stated: ‘It is clear that B. burgdorferi can be transmitted in the blood of infected pregnant women across the placenta into the fetus. This has now been documented with resultant congenital infections and fetal demise. Spirochetes can be recovered or seen in the infant’s tissues including the brain, spleen and kidney..’
Another group of medical experts highlighted the importance of long-term followup of infants exposed to Lyme disease in-utero, stating, "The development of these infants warrants further observation, especially since in another spirochetal infection, congenital syphilis, abnormalities are not always evident at birth."
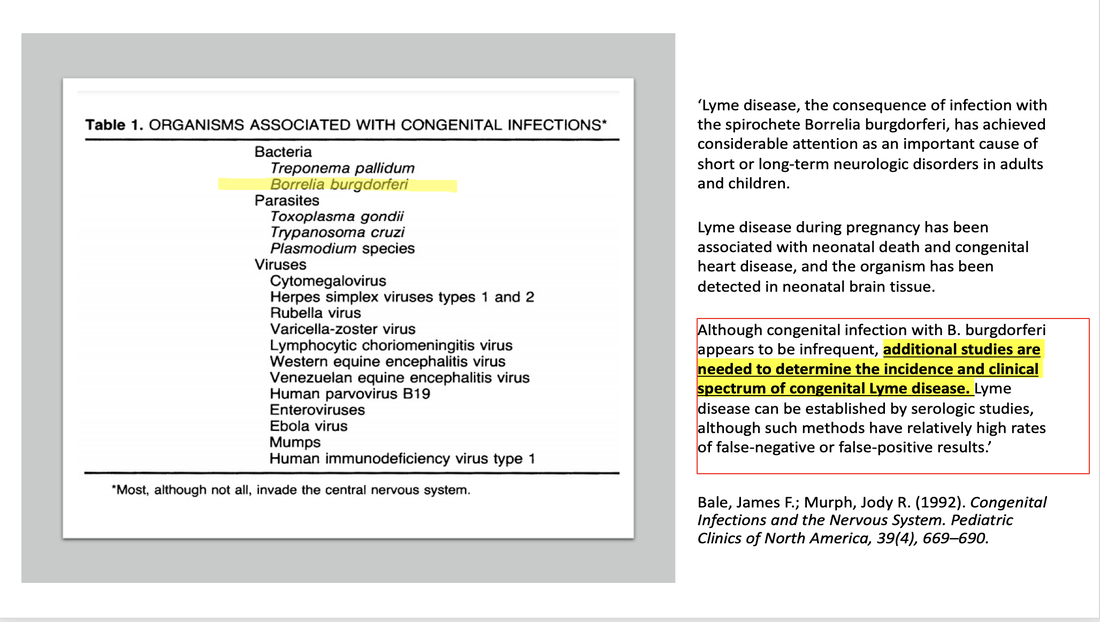
A 1992 publication titled, Current Perspectives on Lyme Borreliosis, authored by Dr. Richard Kaslow from the Epidemiology and Biometry Branch, Division of Microbiology and Infectious Diseases, NIAID (NIH) stated ‘instances of severe illness in infants following transmission from untreated mothers has already lowered the threshold for more aggressive treatment of pregnant woman.’ Another 1992 publication titled Congenital Infections and the Nervous System, authors stated: "Although congenital infection with B. burgdorferi appears to be infrequent, additional studies are needed to determine the incidence and clinical spectrum of congenital Lyme disease.”
A 2015 paper authored by Jasik and colleagues addresses the ability for tick-borne diseases to pass through the placenta of infected animals and humans and the highlights the possibility of intergenerational infection. Regarding Borrelia burgdorferi infection, authors state: ‘It is possible that B. burgdorferi s.l. has a high ability to penetrate mammalian placentae due to its ability of active movement, antigenic and morphological variation, and many other features and causes diagnostic difficulties and problems. In cases of intrauteral fetal infections among patients with Lyme disease, symptoms are not homogeneous. Thus, confirming that B. burgdorferi s.l. is transmitted transplacentally may play important role in the spreading of these pathogens.’ Authors also highlight issues of persistence of infection despite antibiotic treatment and state: ‘The ability of long-term survival of B. burgdorferi s.l. in tissues and spreading of spirochetes in the body despite antibiotic treatment can contribute to intergenerational infection of Lyme disease.’
Several groups of experts authored papers reviewing the clinical aspects and spectrum of Lyme disease, also recognizing congenital infection with Lyme disease. One paper stated, “Like syphilis, Lyme disease during pregnancy may be associated with congenital infection of the infant and resulting clinical illness. Several reports describe infants who died shortly after delivery, or were stillborn, to mothers who had Lyme disease during pregnancy. Spirochetes were found in autopsy specimens from some of these babies.”
A second review titled Clinical Features of Lyme borreliosis listed congenital disease as part of the main clinical spectrum of Lyme borreliosis, also highlighting similarities to syphilis. A third paper by authors who had also reported on the first documented case of transplacental transmission of Bb stated: ‘It is clear that B. burgdorferi can be transmitted in the blood of infected pregnant women across the placenta into the fetus. This has now been documented with resultant congenital infections and fetal demise. Spirochetes can be recovered or seen in the infant’s tissues including the brain, spleen and kidney..’
Another group of medical experts highlighted the importance of long-term followup of infants exposed to Lyme disease in-utero, stating, "The development of these infants warrants further observation, especially since in another spirochetal infection, congenital syphilis, abnormalities are not always evident at birth."
A 1992 publication titled, Current Perspectives on Lyme Borreliosis, authored by Dr. Richard Kaslow from the Epidemiology and Biometry Branch, Division of Microbiology and Infectious Diseases, NIAID (NIH) stated ‘instances of severe illness in infants following transmission from untreated mothers has already lowered the threshold for more aggressive treatment of pregnant woman.’ Another 1992 publication titled Congenital Infections and the Nervous System, authors stated: "Although congenital infection with B. burgdorferi appears to be infrequent, additional studies are needed to determine the incidence and clinical spectrum of congenital Lyme disease.”
A 2015 paper authored by Jasik and colleagues addresses the ability for tick-borne diseases to pass through the placenta of infected animals and humans and the highlights the possibility of intergenerational infection. Regarding Borrelia burgdorferi infection, authors state: ‘It is possible that B. burgdorferi s.l. has a high ability to penetrate mammalian placentae due to its ability of active movement, antigenic and morphological variation, and many other features and causes diagnostic difficulties and problems. In cases of intrauteral fetal infections among patients with Lyme disease, symptoms are not homogeneous. Thus, confirming that B. burgdorferi s.l. is transmitted transplacentally may play important role in the spreading of these pathogens.’ Authors also highlight issues of persistence of infection despite antibiotic treatment and state: ‘The ability of long-term survival of B. burgdorferi s.l. in tissues and spreading of spirochetes in the body despite antibiotic treatment can contribute to intergenerational infection of Lyme disease.’

There is also evidence in research done in animals that Lyme disease can be transmitted in-utero - identified in mice, rats, cows, horses, dogs, coyotes and foxes. Other studies did not find in-utero transmission. A compilation of these studies and references can be found below:
| vertical_transmission_of_bb_in_animals.pdf |
References:
- Schlesinger PA, Duray PH, Burke BA, Steere AC and Stillman MT. Maternal-Fetal transmission of the Lyme disease spirochete, Borrelia Burgdorferi. Ann Intern Med. 1985;103(1):67-8.
- MacDonald A. Gestational Lyme Borreliosis. Implications for the fetus. Rheum Dis Clin North Am. 1989 Nov;15(4):657-77
- Neubert, U. (1987): Erythema migrans in der Gravidität. Hautarzt; 38: 182-183.
- MacDonald AB. Human fetal borreliosis, toxemia of pregnancy and fetal death. Zentralbl Bakt Mikrobiol Hyg A. 1986 Dec;263(1-2):189-200.
- Maraspin V, Cimperman J, Lotric-Furlan, S et al. Erythema migrans in pregnancy. Wein Klin Wochenschr (1999) 111/22-23:933-940.
- MacDonald A, Benach J, Burgdorfer W. Stillbirth following Maternal Lyme Disease. New York State Journal of Medicine vol 87, Nov 1987.
- Lavoie PE, Lattner BP, Duray PH, Barbour AG, Johnson RC. Culture positive seronegative transplacental Lyme Borreliosis infant mortality (1987) Arthritis Rheum, 30(4), 3(suppl):S50
- Weber K, Bratzke H, Neubert UWE et al. Borrelia Burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. Pediatric Infectious Disease Journal Vol 7, No 4, 286-289, 1988
- Horowitz R, Yunker LL. Lyme Disease and Pregnancy: Implications of Chronic Infection, PCR testing and Prenatal Treatment Case Presentation. 16th International Scientific Conference on Lyme Disease and other Tick-Borne Diseases. June 7, 8, 2003.
- Dattwyler R, Volkman D, Luft B. Immunologic aspects of Lyme borreliosis. Review of Infectious Diseases, Vol 11(6) 1989.
- Gasser R, Dusleag J, Reisinger E, et al. A Most Unusual Case of a Whole Family Suffering from Late Lyme Borreliosis for Over 20 Years. Angiology. 1994;45(1):85-86.
- Gardner T. Infectious Diseases of the Fetus and Newborn Infant. In: Remington JS, Klein JO, editors. Lyme Disease, Chapter 11. 5th ed. Philadelphia, PA: The W.B. Saunders Co.;2001. pp. 519-641.
- Lampert, R. Infantile multisystem inflammatory disease: another case of a new syndrome. Eur J Pediatr (1986) 144:593-596.
- Trevison G, Stinco G, Cinco M. Neonatal skin lesions due to a spirochetal infection: a case of congenital Lyme borreliosis? Journal of Dermatology, 36, 677, 1997.
- Lazebnik T, Zal'tsman P. A Case of Congenital Neuroborreliosis. St Petersburg Medical Academy of Postgraduate Education, St. Petersburg, Russia. 2005.
- Jones, CR., Smith H., Gibb E, Johnson L. Gestational Lyme Disease Case Studies of 102 Live Births. Lyme Times, Gestational Lyme Studies, Summer 2005, pp. 36-38.
- Onk, G., Acun C., Murat K., et al. Gestational Lyme disease as a cause of Congenital Hydrocephalus. J Turkish German Gynecol Assoc. Vol 6(2):156-157.
- MacDonald A. Gestational Lyme Borreliosis. Implications for the fetus. Rheum Dis Clin North Am. 1989 Nov;15(4):657-77
- Horst, H.: Borrelia burgdorferi-Infektionen in der Schwangerschaft. In: Hassler, D. (Hrsg): Infection Taschenbuch Lyme-Borreliose. MMV. Medizin Verlag, Munchen 1992, 85-91.
- Vanousova D, Nemcova A, Hulinska D, Schmiedbergerova R., Hercogova J. Transplacentární přenos borelií? Čes-slov Derm, 2007, roč. 82, č. 4, s. 218
- Spector, R., Rummelt, V., Folberg R. The Pathology of Congenital Lyme Borreliosis. Abstract 1466-27. Investigative Opthamology and Visual Science, Annual Meeting. May 2-May 7, 1993, Sarasota Florida. March 15, 1993. Vol 34, No 4.
- Burrascano J (1993) Failure of aggressive antibiotic therapy to protect the placenta from invasion by B. burgdorferi in a pregnant patient with Lyme borreliosis. 6th Annual International Science Conference on Lyme Disease and other Tick-borne Diseases. May 5-6, 1993, Atlantic City, NJ.
- Qureshi MZ, New D, Zulqarni NJ, Nachman S. Overdiagnosis and overtreatment of Lyme disease in children. Pediatr Infect Dis J. 2002 Jan;21(1):12-4.
- Hercogova J, Vanousova D. Syphilis and borreliosis during pregnancy. Dermatol Ther. 2008 May-Jun;21(3):205-9.
- Hulinksa, D., Votypka J., Horejsi J. Disseminated Lyme borreliosis and its laboratory diagnosis. Zpravy Epidemiologie A Mikrobiologie (SZU Praha) 2011:20(1)
- Patmas, M. Letter to the Editor. Persistence of Borrelia burgdorferi despite antibiotic treatment. Journal of Spirochetal and Tick-Borne Disease, Vol 1(4), 1994. Pp. 101.
- Figueroa R, Bracero LA, Aguero-Rosenfeld, M et al. Confirmation of Borrelia Burgdorferi spirochetes by polymerase chain reaction in placentas of women with reactive serology for Lyme antibodies. Gynecol Obstet Invest. 1996;41(4):240-3.
- Nadelman RB, Wormser GP. A clinical approach to Lyme disease. Mt Sinai J Med. 1990 May;57(3):144-56. PMID: 2196462.
- Weber, K. Clinical Features of Lyme Borreliosis. Clinical differences between European and North American Lyme borreliosis – a Review. Stanek (Ed.). Lyme borreliosis II, Zbl Bakt. Suppl. 18, 1989.
- Duray PH, Steere AC. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65-79.
- Kaslow RA. Current Perspective on Lyme Borreliosis. JAMA. 1992;267(10):1381–1383. doi:10.1001/jama.1992.03480100087037
- Trock DH, Craft JE, Rahn DW. Clinical manifestations of Lyme disease in the United States. Conn Med. 1989 Jun;53(6):327-30. PMID: 2667885.
- Luft BJ, Dattwyler RJ. Lyme borreliosis. Curr Clin Top Infect Dis. 1989;10:56-81. PMID: 2679700.
- Bale, James F.; Murph, Jody R. (1992). Congenital Infections and the Nervous System. Pediatric Clinics of North America, 39(4), 669–690.
- Jasik KP, Okła H, Słodki J, Rozwadowska B, Słodki A, Rupik W. Congenital Tick Borne Diseases: Is This An Alternative Route of Transmission of Tick-Borne Pathogens In Mammals? Vector Borne Zoonotic Dis. 2015 Nov;15(11):637-44.
- Johnson, WA. Report on Lyme Disease. Prepared for US Army Corps of Engineers Field Personnel. USAE Waterways Experiment Station, Environmental Laboratory 3909 Halls Ferry Road, Vicksburg, MS 39180-6199. 1993.